Participant Information
The blue buttons below contain links to all you need to know about the SAFE-D study. However, for a quick overview please continue scrolling down.

Who can participate?
You can participate if you have been diagnosed with type 2 diabetes in the last 6 months and are aged between 50 and 84 years old.

What would I have to do?
If you consent to take part, we ask that you attend 3 appointments for a blood test, and to complete a short questionnaire.
What is the purpose of the SAFE-D study?
The purpose of this study is to test a newly developed blood test called Avantect which detects signals of the presence of pancreatic cancer.
You may have been asked if you would like to participate in the SAFE-D clinical research study because you have recently been diagnosed with type 2 diabetes and this is a risk factor for pancreatic cancer. The Avantect test aims to detect the presence of pancreatic cancer when it is at an early more treatable stage.
Why are we talking about pancreatic cancer when I have been diagnosed with diabetes?
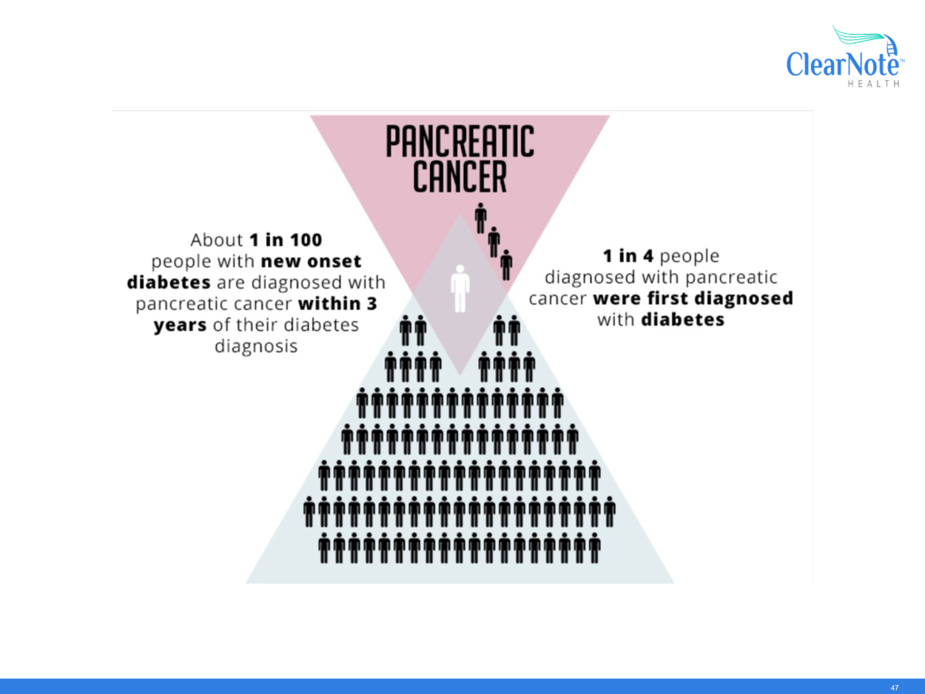
Pancreatic cancer can damage the insulin-producing cells just like in diabetes and can that way be mistaken for being diabetes. People with newly diagnosed type 2 diabetes therefore have a higher-than-normal risk of also being diagnosed with pancreatic cancer within the next 3 years. In the UK, the lifetime risk of being diagnosed with pancreatic cancer is around 2%. Of these, 1 in 4 people are first diagnosed with diabetes. So, while most people with diabetes will not go on to develop pancreatic cancer, this patient group gives us a good opportunity to test how accurate the new blood test is at the same time as ruling out pancreatic cancer earlier.
Why would I need a blood test for pancreatic cancer?
The problem with pancreatic cancer is that it has few obvious symptoms at the early stages, and so it is often only picked up at more advanced stage when the cancer is more difficult to treat. Currently there is no screening test for pancreatic cancer. This new Avantect test is designed to detect the possible presence of pancreatic cancer at an early treatable stage, long before symptoms become apparent.
If the Avantect blood test is proven to accurately detect the presence of pancreatic cancer early enough, it will have life-changing benefits for thousands of people with newly diagnosed diabetes.
It is up to you if you want to take part. If you decide to take part, you are free to stop at any time for any reason, and you will always receive the best possible care available.
Symptoms of pancreatic cancer can be found on the Cancer Research UK website
>Find out more about pancreatic cancer symptoms
What would I be asked to do?
You will be required to fill in a consent form to take part.
You will be asked to attend a total of 3 appointments, 6 months apart at a conveniently located research site, which could be a GP practice or other local medical centre.
During each of these appointments, you will be asked to complete a short questionnaire to provide information about your medical history, and how you are feeling. This should take only a few minutes. A trained healthcare provider will take about 30 ml of blood (roughly 2 tablespoons) from your vein.

What happens next?
Your blood samples will be transferred to a laboratory at University Hospital Southampton for processing and storage.
We need to compare participants who follow the normal standard of care process with those who have their blood tested on the Avantect test.
To do this, half the study participants will be randomly assigned to a Control Group (following standard of care for comparison) and half to an Intervention Group (where the blood samples are tested by the Avantect test).
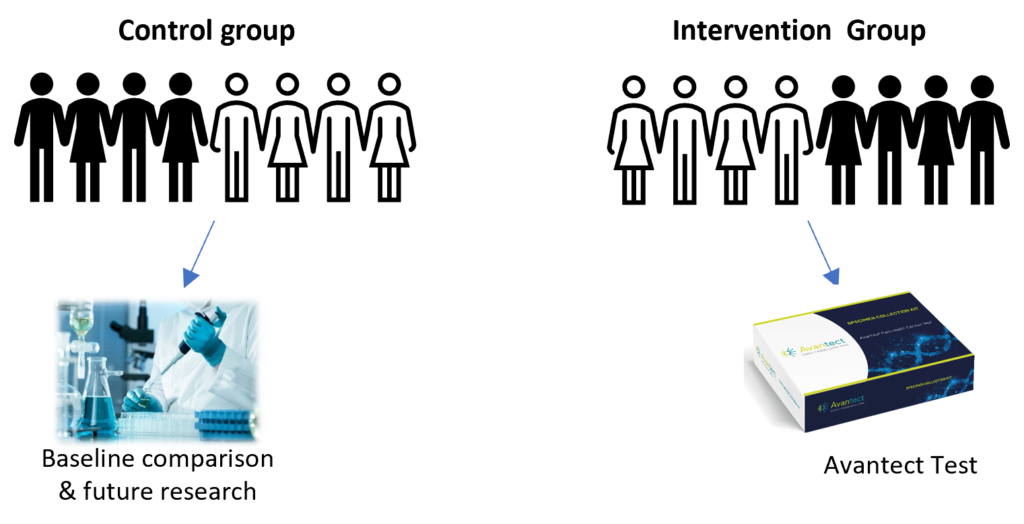
You have equal chance of being listed in either group and you will not know which group you have been assigned to, unless the Avantect test returns a “detected” result.
If you are listed in the Intervention group and a cancer signal is detected with the Avantect test, you will be informed and your GP and the SAFE-D team will arrange a follow-up imaging scan such as MRI or CT scan, which are similar to an x-ray, these take a detailed picture of your pancreas to rule out a pancreatic cancer.
If you do not receive a test result from us, you either have a non-actionable “non-detected” Avantect test result (Intervention group) or you have been listed in the Control group where samples are not run on the Avantect test. It is therefore really important you continue to monitor your own health as normal and inform your healthcare provider of any issues.
Help us improve early pancreatic cancer detection to save lives
