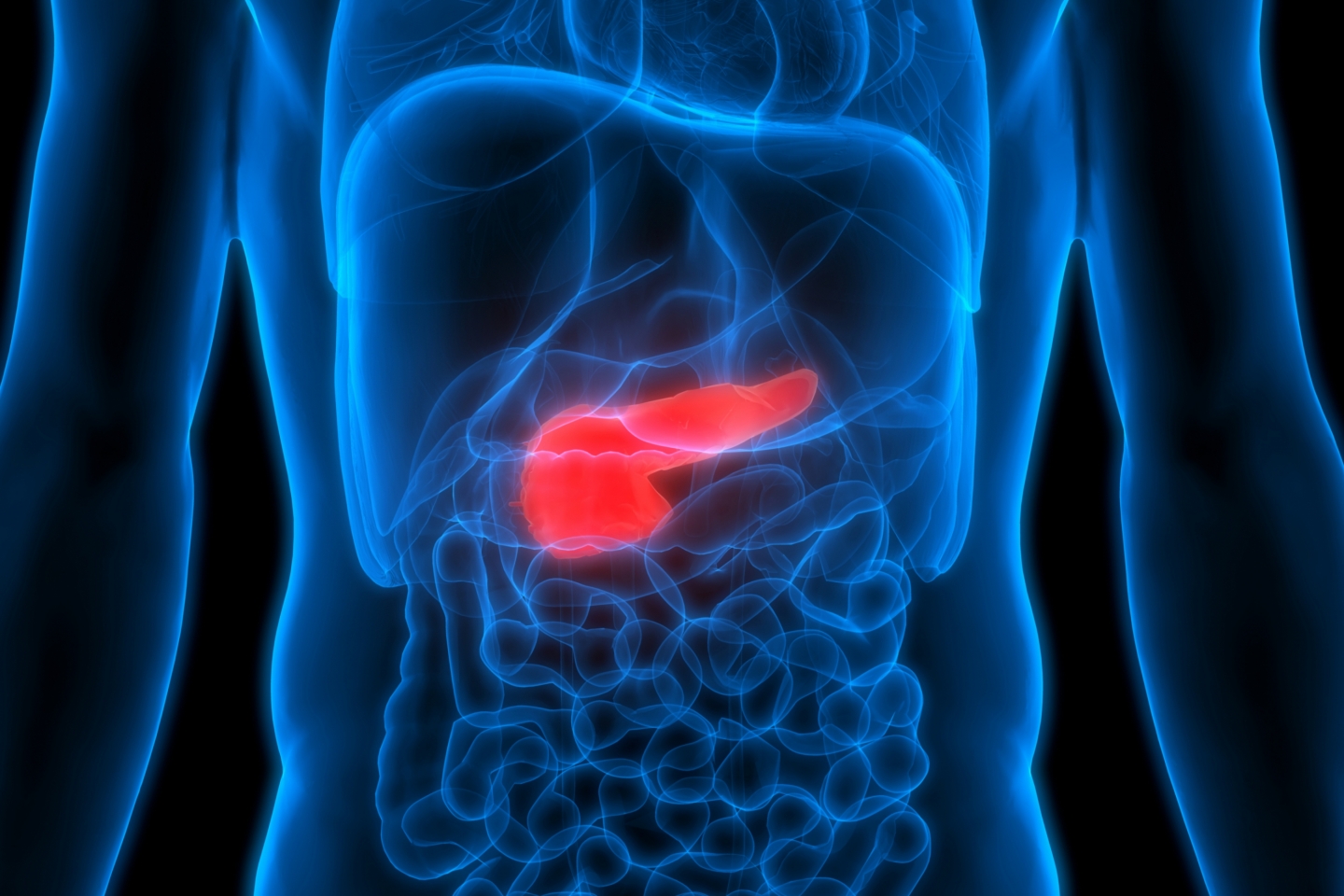
Welcome to the Safe-D study
A clinical study for early detection of pancreatic cancer. Find out more on this website before registering.
What is SAFE-D?
SAFE-D is a clinical research study that aims to test how well a new blood test called Avantect works to detect very small signals of pancreatic cancer at an early stage.
It is known that people recently diagnosed with type 2 diabetes have a slightly higher than normal risk for developing pancreatic cancer. Pancreatic cancer has no obvious symptoms in it’s early stages, making it difficult to diagnose when it is easiest to treat. Unfortunately, at the moment, there is no simple screening test for the disease.
If the Avantect blood test is proven to accurately detect the presence of pancreatic cancer early enough, it will have life-changing benefits for many thousands of people with newly diagnosed diabetes.
If you have been diagnosed with type 2 diabetes within the last 6 months, we would be grateful for your help. Please make an appointment for a blood test at your nearest SAFE-D hub.
Study participation

Who can participate?
You can participate if you have been diagnosed with type 2 diabetes in the last 6 months and are aged between 50 and 84 years old.
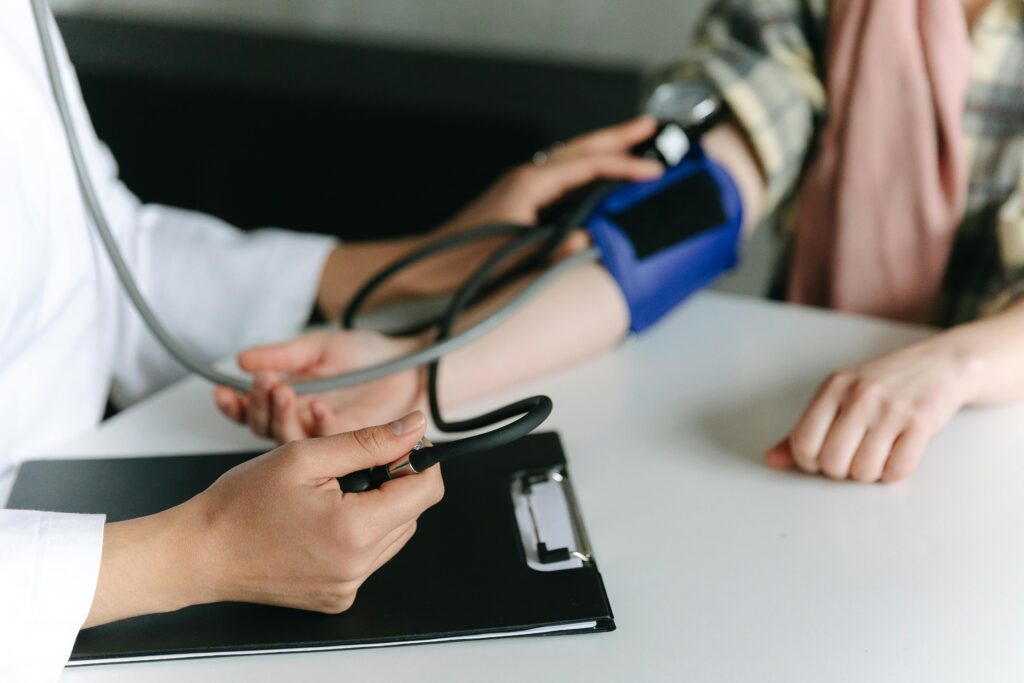
What do I have to do?
If you consent to take part, we ask that you attend 3 appointments for a blood test, and to complete a short questionnaire.
Where can I take part?
You can attend an appointment at a research site of your choice.
About pancreatic cancer
Even if you decide to take part in the SAFE-D study it is always important to be vigilant of any signs of pancreatic cancer and contact your GP for advice.
Symptoms of pancreatic cancer can be found on the Cancer Research UK website and also the Pancreatic Cancer Action website.